Therapeutic Proteins: Types, Design and Production
Therapeutic proteins are associated with unique challenges in terms of stability, delivery and manufacturing complexity.
Therapeutic proteins are a major class of modern pharmaceutical used to treat a wide range of diseases, including cancer, autoimmune disorders, rare genetic conditions and metabolic diseases. Unlike traditional small molecule drugs, which are typically chemically synthesized and interact with defined binding pockets on target proteins, therapeutic proteins are large, biologically derived molecules that mimic or modulate the body’s natural physiological processes. As biologics, they offer several advantages, including high specificity, potent biological activity and the ability to interact with targets that small molecules often cannot reach. However, their development and use are also associated with unique challenges in terms of stability, delivery, immunogenicity and manufacturing complexity. This article introduces the major types of therapeutic proteins, their design strategies and how they are produced at scale.
What are therapeutic proteins?
- Monoclonal antibodies
- Enzymes
- Fc fusion proteins
- Antibody-drug conjugates
- Blood factors
- Cytokines (interleukins and interferons)
- Protein subunit vaccines
- Hormones
What are therapeutic proteins?
In drug development, the term “modality” refers to the category of therapeutic based on its chemical or biological nature. Small molecules are the most established modality, known for their chemical stability, ease of formulation, oral bioavailability and ability to cross cell membranes. But while small molecules are convenient and inexpensive to produce, they are sometimes too simple to achieve the precision or selectivity required for complex diseases. In particular, small molecules struggle to disrupt large, flat protein-protein interactions and often affect multiple off-target pathways, which can lead to side effects.1
Therapeutic proteins, on the other hand, are typically based on or inspired by naturally occurring proteins in the human body. These include antibodies, enzymes, cytokines and hormones, among others (Table 1). Their size and complexity give them several advantages over small molecules, especially when it comes to targeting extracellular or membrane-associated proteins, modulating immune responses or replacing missing or malfunctioning proteins in patients. Due to their structural similarity to endogenous proteins, therapeutic proteins often provide better specificity, reduced off-target effects and the potential for more natural regulatory effects. However, they cannot be taken orally because they are digested; instead, they must be delivered by injection or infusion. Additionally, they are more susceptible to degradation and denaturation during storage and circulation.2
Types of therapeutic proteins
There are many different types of therapeutic proteins, each with distinct functions and therapeutic applications. The major categories include monoclonal antibodies, enzymes, Fc (fragment crystallizable) fusion proteins, antibody-drug conjugates, blood factors, cytokines, subunit vaccines and hormones.
Monoclonal antibodies
Monoclonal antibodies (Figure 1) are one of the most widely used types of biologic drugs. They are designed to bind with high specificity to a single target, known as an antigen. These antibodies are created by cloning a single type of immune cell so that all the antibodies produced are identical. This high specificity allows monoclonal antibodies to recognize and bind only to disease-related targets, such as cancer cell markers or inflammatory proteins, while avoiding healthy tissues.3
Some monoclonal antibodies work by directly blocking the activity of a harmful molecule, while others flag diseased cells for destruction by the immune system. Due to their precision, monoclonal antibodies are used to treat a variety of conditions, including cancers, autoimmune disorders and infections.4
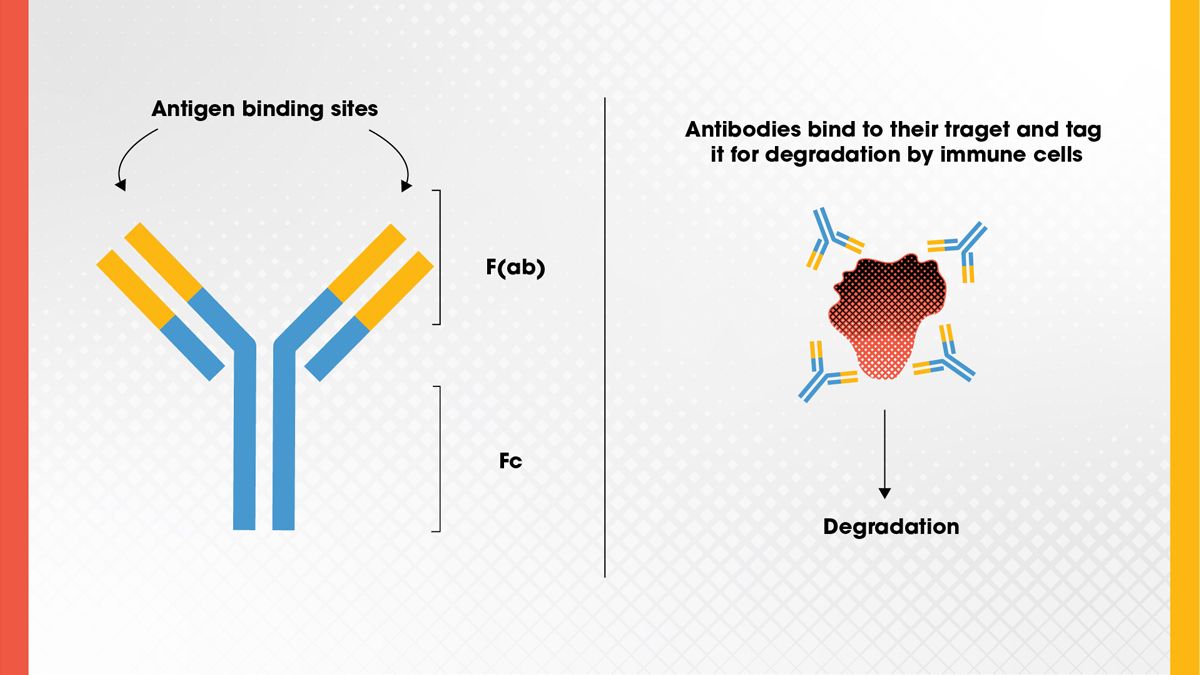
Figure 1. Antibody structure, showing the antigen binding site and F(ab) and Fc regions, and the basic function of the antibody. Credit: Technology Networks.
Enzymes
Therapeutic enzymes are proteins that catalyze specific biochemical reactions. In the context of disease treatment, they are often used to replace enzymes that are missing or defective in patients with genetic disorders. For example, in lysosomal storage diseases, such as Gaucher or Pompe disease, enzyme replacement therapy can help break down accumulated toxic substances in cells. 5
Enzymes can also be used to aid digestion in patients with pancreatic insufficiency or to target tumor cells by converting prodrugs into active drugs. Because enzymes are highly specific in their action, they can be utilized to carry out precise therapeutic functions.
Fc fusion proteins
Fc fusion proteins are engineered drugs that consist of a biologically active protein domain fused to the Fc region of an antibody (Figure 2). This fusion takes advantage of the Fc region’s natural ability to extend the half-life of antibodies in the bloodstream by binding to receptors that recycle antibodies rather than degrading them.6
This design allows Fc fusion proteins to stay in the body longer and perform their therapeutic function over extended periods. They are often used in autoimmune diseases and inflammatory conditions. The first therapeutic Fc-fusion protein was used to treat AIDS.7
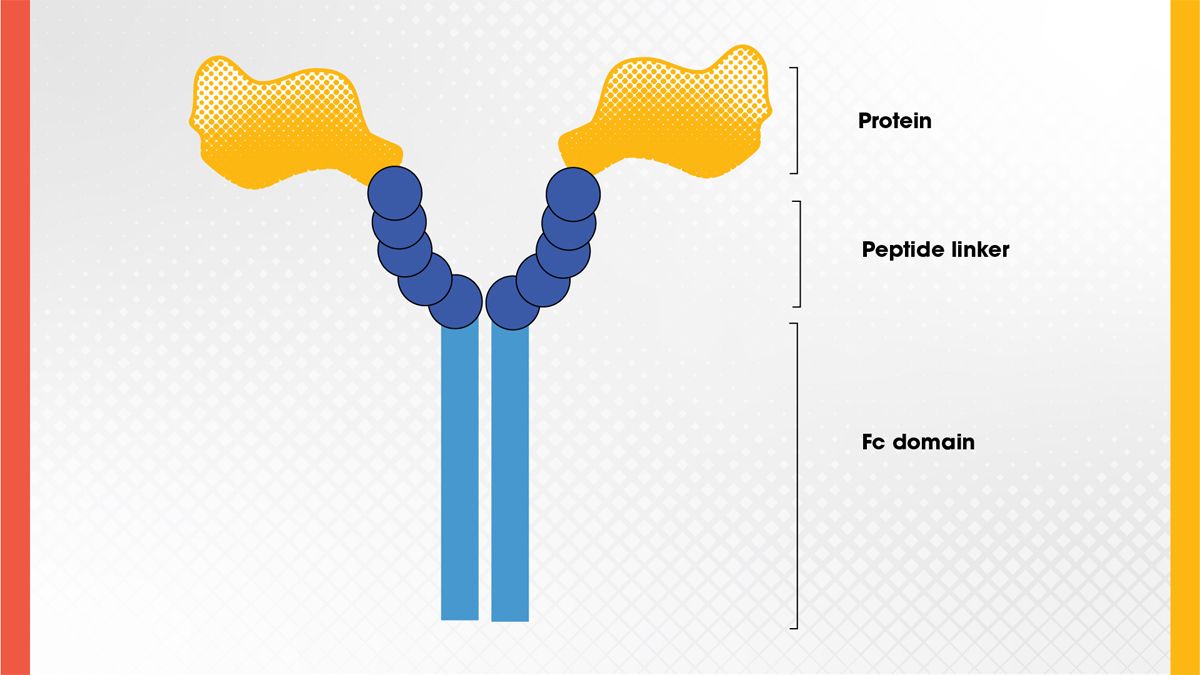
Figure 2. Structure of an Fc fusion protein. Credit: Technology Networks.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are complex molecules that combine the targeting ability of monoclonal antibodies with the potency of cytotoxic drugs (Figure 3). The antibody component guides the drug to a specific cell type, such as a cancer cell, and the drug, or payload, is released upon binding or internalization. This approach enables highly targeted delivery of chemotherapy agents, minimizing damage to healthy tissues.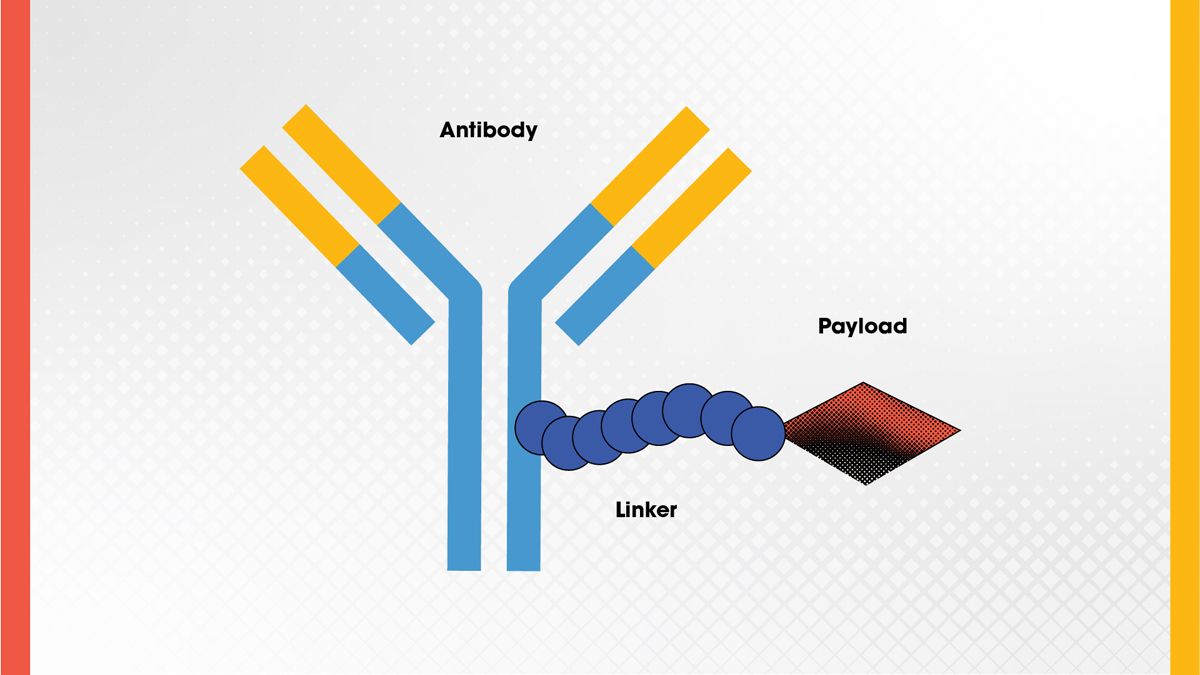
Figure 3. ADC structure showing the antibody, linker, and drug (payload). Credit: Technology Networks.
Blood factors
Blood factors, or coagulation proteins, are essential for stopping bleeding by helping the blood to clot properly. These proteins, such as Factor VIII or Factor IX, are often missing or faulty in people with bleeding disorders, including hemophilia. 11 , 12 To treat these conditions, patients receive replacement therapies made from either donated plasma or recombinant proteins produced in the lab. Some of these therapies are now enhanced, for example, by being fused with other proteins to last longer in the bloodstream. Though mainly used for bleeding disorders, researchers are exploring their use in surgeries, trauma care and rare clotting conditions.Cytokines (interleukins and interferons)
Cytokines are small signaling proteins that modulate the immune system. Interferons are known for their antiviral activity, while interleukins regulate immune cell communication and activation. 13 Therapeutic cytokines are used in conditions such as multiple sclerosis, chronic viral infections and cancer. 14 While mainly used for immune-related conditions, research continues into expanding their applications in areas like infectious disease and immunotherapy.Protein subunit vaccines
Subunit vaccines use purified pieces of a pathogen, typically proteins, to stimulate an immune response without introducing live pathogens (Figure 4). This approach is safer for immunocompromised individuals and has been successfully used in vaccines for hepatitis B, human papillomavirus (HPV) and more recently, COVID-19. 15 , 16 Scientists can design the protein subunits to trigger a strong immune response while minimizing side effects. As technology advances, protein subunit vaccines are being explored for a wider range of diseases, even some cancers. 17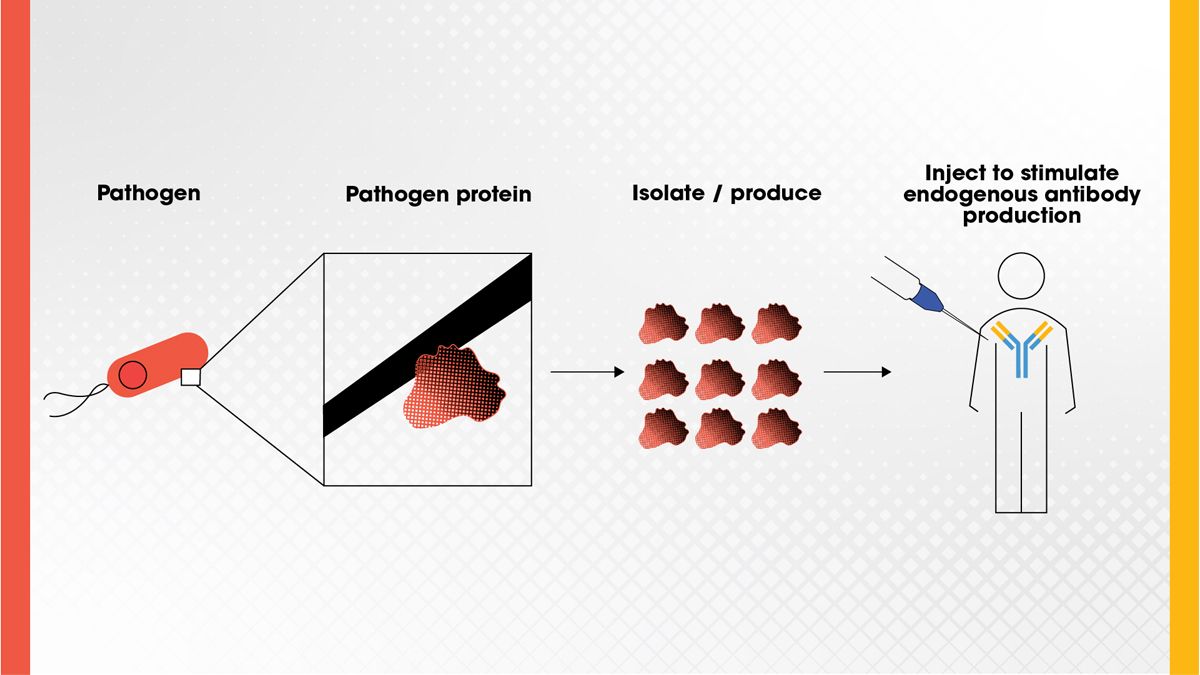
Figure 4. A protein found on a pathogen can be isolated and injected as a vaccine to stimulate antibody production in the body to confer immunity to the pathogen. Credit: Technology Networks.
Hormones
Hormones are a major class of chemical messengers in the body, and when natural levels fall out of balance, lab-made hormones can help restore normal function. These therapies are commonly used in conditions like diabetes, thyroid disorders and hormone deficiencies. For example, insulin helps regulate blood sugar (Figure 5), and synthetic thyroid hormone supports metabolism in people with hypothyroidism. 18 , 19 Researchers are now pushing hormone therapies further, exploring their potential in areas such as cancer treatment, reproductive health and age-related diseases. 20 , 21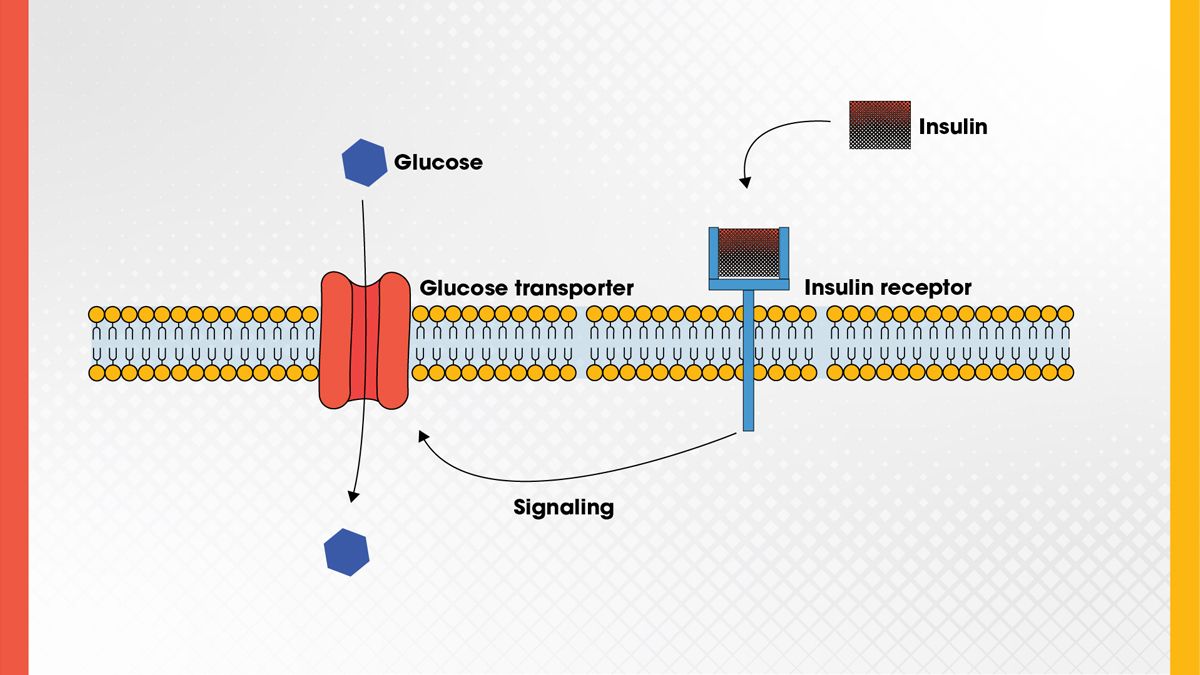
Figure 5. Insulin regulates blood sugar by binding to the insulin receptor, which induces intracellular signaling that activates the glucose transporter to stimulate glucose uptake. Credit: Technology Networks.
Table 1. Examples of protein therapeutics approved by the US Food and Drug Administration and the European Medicines Agency.
| Category | Example | Used For | How it Works |
| Monoclonal antibody | Herceptin™ (trastuzumab) | HER2-positive breast cancer | Targets cancer cells with the HER2 protein, sparing normal cells |
| Enzyme | Myozyme™ (alglucosidase alfa) | Pompe disease | Replaces a missing enzyme to prevent toxic buildup in cells |
| Fc fusion protein | Enbrel™ (etanercept) | Rheumatoid arthritis | Blocks inflammation and lasts longer in the body due to the Fc region |
| Antibody-drug conjugate | Adcetris™ (brentuximab vedotin) | Hodgkin lymphoma | Delivers a toxic drug directly to cancer cells for targeted killing |
| Blood factor | Eloctate™ (efmoroctocog alfa) | Hemophilia A | Replaces clotting protein (Factor VIII) to stop bleeding |
| Cytokine (interferon/interleukin) | Avonex™ (interferon beta-1a) | Multiple sclerosis | Modulates immune activity to slow nerve damage |
| Protein subunit vaccine | Gardasil 9™ (human papillomavirus 9-valent vaccine, recombinant) | HPV prevention | Uses a viral protein to train the immune system without risk of infection |
| Hormone | Lantus™ (insulin glargine) | Diabetes | Regulates blood sugar by replacing natural insulin |
Therapeutic protein design
Designing effective therapeutic proteins involves optimizing a range of properties: target specificity, biological activity, stability, pharmacokinetics and immunogenicity. Several established strategies are utilized to improve these parameters, and emerging methods are pushing the boundaries of what therapeutic proteins can achieve. These innovations reflect a growing ability to rationally engineer complex protein behaviors and to tailor biologics to specific disease contexts with unprecedented precision.
Well-established strategies
- Site-specific mutagenesis: By changing specific amino acids in a protein’s sequence, scientists can improve how the protein functions or behaves in the body. This includes making it more stable, less immunogenic or better at binding to its target.22 This is a basic tool in modern biotechnology.
- PEGylation: Attachment of polyethylene glycol (PEG) chains to proteins increases their size, shields them from immune detection and slows renal clearance, thereby extending circulation time.22
- Fc fusion: A therapeutic protein can be fused to the Fc region of an antibody to extend its half-life and improve its stability. The Fc region interacts with a recycling receptor in the body, allowing the fusion protein to avoid degradation.22
- Glycoengineering: Proteins often have sugar molecules (glycans) attached, and changing these patterns can influence how the immune system responds or how long the protein stays active. Glycoengineering allows scientists to improve protein folding, enhance receptor binding and reduce immunogenicity. This is especially important for antibodies and other heavily glycosylated proteins.22
- Lipidation: Adding lipid (fatty acid) chains to a protein helps it stick to serum albumin, which circulates in the blood for long periods. This delays clearance and helps maintain steady drug levels over time. It’s commonly used in modified insulin and hormone therapies.22
- Albumin fusion or binding: Therapeutic proteins can be fused directly to albumin or engineered to bind it non-covalently. Since albumin has a naturally long half-life, this strategy prolongs the circulation time of the drug. It also improves the protein’s stability and solubility.22
- Disulfide bond engineering: Disulfide bonds help maintain the 3D structure of a protein, and scientists can introduce new ones to enhance stability. This is especially useful for proteins that might unfold or degrade in harsh environments, such as the bloodstream. The approach supports shelf-life and activity under stress.22
Emerging and advanced strategies
- XTENylation/PASylation: These are genetically encoded, biodegradable alternatives to PEGylation, using long, unstructured polypeptides. They extend a protein’s half-life by increasing its size and reducing kidney clearance.23 Unlike PEG, these methods don’t rely on synthetic polymers and are less likely to cause long-term side effects.
- Multispecific proteins (bi- or tri-specifics): These engineered proteins are designed to bind multiple targets simultaneously. In cancer therapy, they might link a T cell or natural killer cell to a tumor cell to trigger a direct immune attack. This improves precision and can overcome resistance seen with single-target drugs.24
- Conditional activation (prodrug proteins): Some proteins are designed to be activated only under specific conditions, such as low pH in tumors or the presence of certain enzymes. This reduces systemic toxicity and increases on-target activity. It’s a promising approach to increase safety without sacrificing potency.25
- Self-assembling protein nanostructures: Engineered proteins can be designed to form larger structures, such as cages or nanoparticles. These structures can carry therapeutic agents or stimulate the immune system more effectively. They are especially promising in vaccines and targeted drug delivery.26
- Glycan masking and steering: By strategically placing sugar groups on a protein in a more targeted use of glycoengineering, scientists can hide regions that might trigger an immune response or alter how the protein moves through the body. This can improve safety and biodistribution and is a precise way to control protein interactions without changing the core sequence.27
- Engineered scaffolds (non-antibody formats): Instead of relying on traditional antibodies, researchers are developing smaller, more stable binding proteins like DARPins, Affibodies and anticalins.28, 29, 30 These scaffolds can be customized for tight, specific binding and are easier to produce. They open new possibilities in diagnostics and therapy.
Computational protein design
Computational tools have become a powerful part of designing therapeutic proteins. Instead of relying only on trial-and-error experiments in the lab, scientists can now use computer models to predict how changes in a protein’s sequence will affect its structure, stability or how well it binds to a target. This approach, known as computer-aided design (CAD), uses software such as Rosetta to simulate protein folding and interactions based on physical and chemical principles. These simulations can help researchers identify the best places to modify a protein to improve its function or reduce unwanted side effects.31, 32
A key success story in this space is Neo-2/15, a synthetic protein designed to act like the interleukins IL-2 and IL-15. Using Rosetta, scientists engineered Neo-2/15 to keep its immune-activating properties while avoiding the part of the IL-2 receptor that causes harmful side effects. In preclinical studies, this engineered cytokine showed strong anti-tumor activity and was safer than natural IL-2.33
More recently, artificial intelligence (AI) has taken protein design to a new level. AI programs such as AlphaFold can predict a protein’s 3D structure from just its amino acid sequence with remarkable accuracy. Knowing a protein’s shape is crucial for understanding how it works and how to improve it. This has made the design process faster and more precise.34
Biotech companies such as Generate:Biomedicines and Evozyne are now using AI to create entirely new proteins for specific medical needs, such as antibodies or enzymes tailored to certain diseases. This shift toward data-driven design accelerates discovery, reduces development costs and opens the door to highly customized biologics that were previously out of reach through traditional methods.
Production processes of therapeutic proteins
Before the advent of recombinant DNA technology in the 1980s, therapeutic proteins were primarily harvested from natural sources such as animal organs, human blood or urine. Early examples include insulin extracted from pig or cow pancreas and clotting factors purified from pooled human plasma. While most therapeutic proteins are now produced recombinantly to ensure purity, consistency and safety, some naturally sourced products are still in use, particularly when recombinant versions are difficult to manufacture or not widely available. These include plasma-derived Factor VIII, immunoglobulins, albumin, urine-derived gonadotropins and porcine pancreatic enzymes.2, 35
Recombinant production typically begins by inserting the gene for the desired protein into a host cell line such as Escherichia coli (E. coli), yeast (Pichia pastoris), mammalian cells (e.g., Chinese hamster ovary cells) or insect cells. The choice of system depends on the complexity of the protein. For instance, while E. coli offers speed and cost-effectiveness, mammalian cells are preferred for proteins that require human-like folding and glycosylation. After small-scale optimization in lab flasks or bench-scale bioreactors, the process is scaled up to large bioreactors, often ranging from 100 liters to 20,000 liters, with strict control over environmental conditions.
Once expressed, the protein is harvested from the culture medium and purified through a series of chromatographic steps, such as Protein A affinity (for antibodies), ion exchange and size exclusion, to remove contaminants and ensure product quality. The final formulation includes stabilizers to maintain activity during storage and delivery. Throughout production, strict adherence to Good Manufacturing Practice (GMP) guidelines is essential to ensure clinical-grade safety, efficacy and consistency.36
Therapeutic protein success criteria and challenges
The success of a therapeutic protein hinges on meeting critical criteria for efficacy and safety. It must bind its target with high specificity and show clear therapeutic benefit in disease models and clinical trials. Equally important, it must avoid significant immunogenicity, toxicity, off-target effects or disruption of normal physiology. These factors are typically assessed in preclinical and early clinical studies. Product quality, including purity, consistency and stability, is crucial to success and is verified through analytical testing under GMP. Regulatory authorities require detailed characterization of the protein’s structure, aggregation state and post-translational modifications before approval.36, 37
Despite advancements, several challenges remain in therapeutic protein development, one of which is immunogenicity. Minor changes in protein structure, glycosylation or impurities can trigger immune responses that neutralize efficacy or cause adverse effects, especially with repeat dosing. Developers rely on predictive algorithms, in vitro assays and rational design, such as antibody humanization or epitope deimmunization, to reduce this risk.38, 39 Other challenges include improving delivery to difficult tissues, like the brain, and ensuring stability during storage.