Cellular Agriculture: Techniques and Applications
A revolution in the production of food and materials
Cellular agriculture is revolutionizing the production of food and materials by using cells instead of whole organisms, offering more sustainable, safe and ethical alternatives. This article explores the techniques, applications and potential of cellular agriculture, focusing on its impact on food production and material innovation.
What is cellular agriculture?
How does cellular agriculture work? Tissue engineering meets synthetic biology
Culturing animal and plant cells – including cultured meats
Culturing microorganisms – precision fermentation
Growth media considerations
Bioreactors for scaling up production
The role of 3D bioprinting in cellular agriculture and 3D printed food
Pros and cons of cellular agriculture
Applications of cellular agriculture, current state and future perspectives
- Lab grown meat
- Other food-based alternative proteins
- Vegan leather
- Artificial silk
- Other applications
What is cellular agriculture?
Cellular agriculture is an emerging field that focuses on the production of agricultural products directly from cell cultures instead of relying on whole animals or plants. These product can be divided into two categories: cellular products (like cultured meat, leather or seafood, produced from actual animal and plant cells) and acellular products (like milk proteins or egg whites, produced from cultures of microorganisms – e.g., bacteria, yeasts, fungi and algae – via fermentation).1
Cellular agriculture combines several scientific fields, including biotechnology, molecular biology, tissue engineering and synthetic biology, to create sustainable alternatives to conventional farming. It aims to produce agricultural products that closely replicate the molecular, sensory, nutritional and structural qualities of those made through conventional methods. The benefits include reduced environmental impact, less land and water use, fewer greenhouse gas emissions2 and ethical advantages by eliminating large-scale animal farming and reducing disease risks.3 It also provides greater food security with controlled production and allows precise control over nutritional content.4
The concept dates back nearly a century. In 1932, Winston Churchill famously predicted that humans would one day grow only the edible parts of animals, envisioning a future where meat could be produced without raising whole animals. However, it wasn’t until the 21st century that this concept became real, driven by accumulated scientific knowledge and technological innovations. In 2013, the world saw the first lab-cultured hamburger, grown from cow muscle cells by Dr. Mark Post and his team at Maastricht University.5 Since then, cellular agriculture has transformed from a scientific curiosity to a rapidly developing field, attracting billions in investment and leading to the creation of many startups, research labs and pilot manufacturing facilities around the globe.6,7 An overview of the general cellular agriculture process for cultured meat production is presented in Figure1.
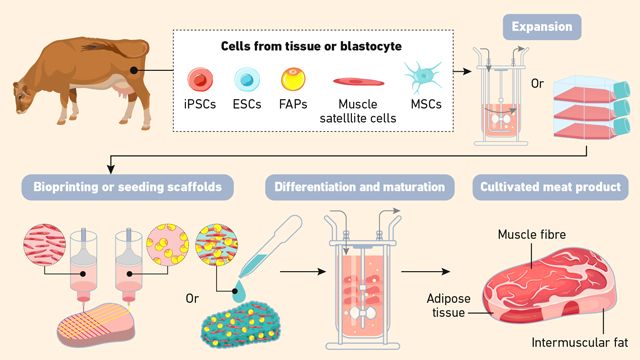
Figure 1: Overview of the general cellular agriculture process for cultured meat production. Credit: Technology Networks.
How does cellular agriculture work? Tissue engineering meets synthetic biology
At the core of cellular agriculture are two main approaches:
- Cell culture-based production: This uses tissue engineering to grow animal or plant cells directly into structured products, such as meat or leather.
- Fermentation-based production: This uses naturally occurring or genetically engineered microbes to produce specific proteins, fats and other biomolecules.
Culturing animal and plant cells – including cultured meats
Culturing animal and plant cells involves isolating specific cell types – such as muscle satellite cells or plant parenchyma cells – and growing them in vitro under controlled conditions, in bioreactors.8 Plant cells can be used in cellular agriculture, primarily for producing rare plant-based ingredients (e.g., saffron, vanilla or ginseng), pigments or biopharmaceuticals. However, it plays a minor role compared to animal cell culture or precision fermentation due to limited efficiency and scalability.
The process of creating cultured meat or other tissue-based products typically begins with the selection and cultivation of specific cell types (Figure 1). In the case of meat, satellite cells – a type of muscle stem cell – are isolated from a living animal. These cells are chosen for their ability to proliferate and differentiate into mature tissue types like muscle and fat. Once isolated, the cells are placed in a growth medium that provides the necessary nutrients and growth factors to support cell proliferation. As the cells multiply, they are transferred onto scaffolds – three-dimensional (3D) structures that resemble natural tissues – into the bioreactor. Scaffolds are biocompatible frameworks made from natural or synthetic materials (e.g., collagen, alginate, cellulose). They not only provide a physical surface for cells to adhere to and grow upon but also help replicate the texture, mouthfeel and appearance of natural cellular tissue.9 Advanced methods like 3D bioprinting may be used to layer cells in specific patterns, mimicking the texture and complexity of animal meat.1
Cultured meat is starting to be approved in a few countries, sustained by scientific, regulatory, ethical and economic factors. Singapore, the U.S. and Israel have allowed cell-based meat, such as cultivated chicken, and the UK has approved it for pet food.8
Culturing microorganisms – precision fermentation
Culturing microorganisms is a foundational method in cellular agriculture, particularly for producing animal-free food ingredients, materials and functional compounds. This approach leverages microbes (like yeast, bacteria or fungi) to use the whole biomass or produce target compounds. Fungal culture is a powerful method in cellular agriculture and alternative protein production, where filamentous fungi are cultivated to produce high-protein food ingredients. This process involves growing fungi in controlled fermentation tanks, feeding them with nutrients and harvesting the biomass for use in food products. Quorn® is one of the most well-known and widely consumed products made from fungal mycoprotein. Another established line is microalgal cultivation to produce food, feed or high-value compounds without relying on traditional farming. Microalgae can be used as food supplements or for the production of protein-rich ingredients for alternative meat and dairy. Organisms such as spirulina and chlorella are used for natural food coloring, and some microalgal strains can also produce omega-3 oils used as fish oil replacements. Microalgal cultivation is a growing branch of cellular agriculture, especially in respect to producing alternative proteins, lipids or functional food ingredients through cell-based cultivation.10
Precision fermentation is an emerging biotechnology process used to produce specific functional ingredients by programing microorganisms (Figure 2). It involves the insertion of specific genes to modify microorganisms, such as yeast, bacteria or fungi to produce specific biomolecules, including proteins, fats, pigments and enzymes, which can improve the quality, flavor, safety and sustainability of foods.9 By harnessing this technology, food producers can create more consistent and natural ingredients while reducing reliance on animal-based products and minimizing environmental impacts. It offers a promising solution for enhancing food production, making it more efficient and eco-friendly, while also ensuring safer and more nutritious products.11
The process starts by inserting a gene encoding the desired protein into the genome of a microorganism. These engineered microbes are then cultivated in bioreactors – fermentation tanks – where they metabolize sugars and other nutrients and produce the target biomolecules. After fermentation, the protein is extracted, purified and processed into a variety of products.12
Unlike cell-cultured tissue products, fermentation-based outputs are typically acellular – they don’t involve the cultivation of animal cells but instead focus on replicating the biochemical components of animal products. Currently, there are a number of companies producing animal-free dairy proteins, such as whey, casein and egg white protein and even collagen for leather.12
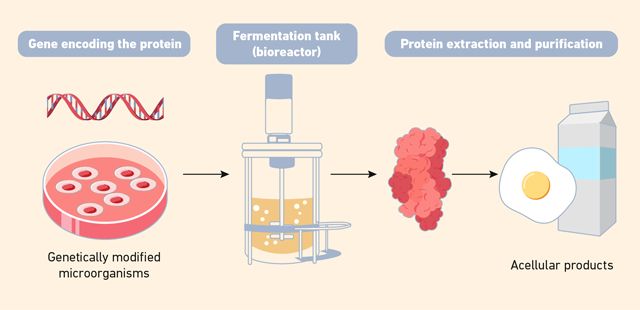
Figure 2: Schematic representation of the production of acellular products via precision fermentation. (Microorganisms are genetically engineered to produce specific proteins and compounds, which are then harvested without using animal cells.) Credit: Technology Networks.
Growth media considerations
Growth media is essential in cellular agriculture, as it provides cells with nutrients such as amino acids, glucose, salts and vitamins. These compounds support the growth, division and specialization of the cells. In traditional cell culture, fetal bovine serum (FBS) was often used due to its rich composition of growth factors and nutrients essential for cell proliferation. However, concerns over ethical issues, batch variability, contamination risks and high costs have driven the search for alternatives.13 Cellular agriculture aims to use serum-free, animal-free, antibiotic-free and plant-based media that are more sustainable and scalable. These new media include plant hydrolysates, recombinant proteins (produced via precision fermentation), synthetic media formulations and optimized nutrient blends tailored to specific cell types.14 In addition to being more in line with environmental and animal welfare goals, these improvements significantly reduce costs and increase the scalability for large-scale manufacturing. A comparison of traditional and cellular agriculture media is shown in Table 1.
Table 1: A comparison of growth media used in traditional cell culture and cellular agriculture.
| Traditional Media (FBS) | Cellular Agriculture Media | |
| Source | Animal-derived (fetal bovine serum) | Plant-based, synthetic or recombinant |
| Composition | Naturally rich in growth factors and nutrients | Customizable, defined blends (plant hydrolysates, proteins) |
| Ethical Concerns | High – involves animal slaughter | Low – animal-free, cruelty-free |
| Batch Consistency | Variable between batches | More consistent and controlled |
| Contamination Risk | Higher – risk of pathogens and impurities | Lower – sterile and defined components |
| Cost | High and increasing | Potentially lower with scale |
| Scalability | Limited by supply of FBS | Designed for large-scale, sustainable production |
| Suitability for Cell Types | Broad, but not tailored | Can be optimized for specific cell types |
Bioreactors for scaling up production
To meet market demand, cellular agriculture must scale up from laboratory- to industrial-scale production using bioreactors. These are controlled vessels that maintain optimal conditions – such as temperature, pH, oxygen and nutrient levels – to support consistent and efficient cell growth. Bioreactors allow cells to be mixed, agitated and aerated to ensure proper distribution of nutrients and gases throughout the culture.1
The production process of cellular agriculture typically begins with cell expansion, which involves a sequence of bioreactors of increasing volume. This setup helps maintain exponential cell proliferation while preventing premature differentiation. During this stage, stirred-tank bioreactors are commonly used for culturing animal cells in suspension. They offer effective mixing, oxygen transfer and foam management, all of which are crucial for supporting both the growth and eventual differentiation of stem or progenitor cells. Once a sufficient number of cells has been cultivated, they are attached to scaffolds or arranged into 3D structures through 3D bioprinting. These structures are then transferred into perfusion bioreactors, which are specifically designed for the maturation phase. Here, the cells develop into functional tissue – such as muscle or fat – under precisely controlled flow conditions that support nutrient delivery, waste removal and tissue structuring.15 A representative diagram of the whole process is presented in Figure 3.
In precision fermentation, a similar stirred-tank bioreactor setup is used, often incorporating microcarriers—tiny spherical particles that increase the available surface area for cell growth. This approach focuses on optimizing microbial productivity, allowing engineered microorganisms to produce target proteins, enzymes or fats efficiently, while also simplifying the process of downstream purification and extraction.16
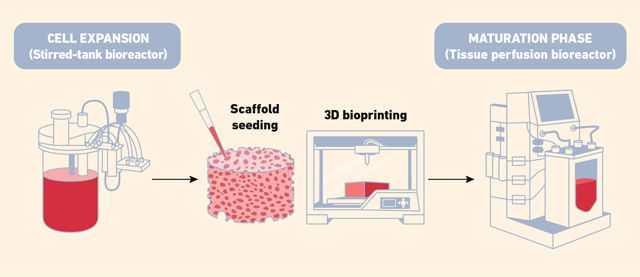
Figure 3: Bioreactors used for scaling up production in cellular culture. (These systems support controlled cell growth and tissue development under optimized conditions for large-scale manufacturing.) Credit: Technology Networks.
The role of 3D bioprinting in cellular agriculture and 3D printed food
In cellular agriculture, 3D bioprinting is used to manufacture structured and realistic food products, such as cultured meat, through the layering of cells and biomaterials in precise, programable patterns. By using this method, it is possible to mimic the composition, texture and appearance of typical meat cuts, including the appearance of fat marbling and muscle fibers.17
A crucial element in this process is the scaffold, which offers a 3D structure that facilitates tissue development, cell adhesion and growth. Scaffolds can appear in the form of hydrogels, fiber scaffolds and microcarriers. These can be produced via 3D printing or extrusion methods, which is further subdivided into fused deposition modeling (FDM) and extrusion modeling (EM). Scaffolds are made from a range of materials, such as synthetic or bioengineered materials (like polylactic acid (PLA) or alginate), animal-derived proteins (like collagen or gelatin) and edible plant-based polymers (like cellulose or soy protein). Each variety has unique mechanical strength, porosity, biodegradability and biocompatibility characteristics. Furthermore, the ideal scaffold should facilitate the flow of nutrients and oxygen, promote cell development and be safe for consumption.18
In addition to creating structured meats like steaks, burgers and fillets, bioprinting also facilitates personalized nutrition, adapting food products to meet specific consumer preferences and needs.19
Pros and cons of cellular agriculture
Cellular agriculture has great potential to produce animal-based products and alternatives in a sustainable and ethical way. But despite its many advantages, several challenges remain unsolved. Weighing the pros and cons is essential to ensure responsible development, public trust and comprehensive food system improvement. Understanding both sides helps to guide smart policy, investment and research decisions for a more ethical and resilient food future.
Pros
- Environmental sustainability: Reduces greenhouse gas emissions, water usage and land use.
- Animal welfare: Eliminates the need for raising and slaughtering animals.
- Food security: Offers a stable food supply that is less vulnerable to climate change or pandemics.
- Customization: Enables tailored nutrition and functionality.
- Scalability: Potential for mass production with fewer natural resources.
Cons
- High costs: Still relatively expensive compared to conventional products.
- Regulatory hurdles: Unclear regulations in many regions.
- Consumer acceptance: Skepticism about safety and naturalness.
- Technical challenges: Difficulty replicating complex tissue structures/textures and flavors.
- Economic and social changes: Redefinition of farming and farmers’ way of life.
Applications of cellular agriculture, current state and future perspectives
Cellular agriculture spans from traditional uses in health and alternative foods to cutting-edge applications in animal free products such as meat and leather (Figure 4). Animal cells and microorganisms can be cultivated in controlled environments, offering a clean, efficient alternative to conventional farming and animal agriculture. These practices are central to building a sustainable, ethical and innovative food system. Whether through more established practices like fermenting algae and fungi, or modern advances like precision fermentation, they offer scalable solutions to reduce our dependence on animals, lower environmental impact and meet growing global food demand.
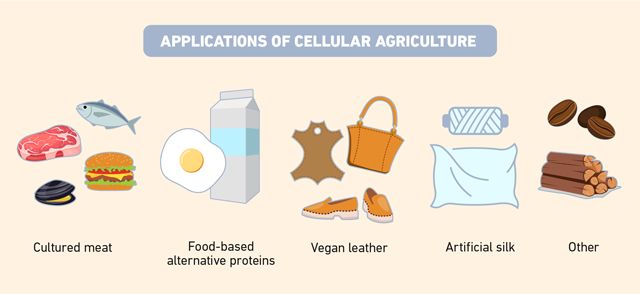
Figure 4: Examples of applications of cellular agriculture. Credit: Technology Networks.
Lab grown meat
Cellular agriculture uses tissue engineering to grow animal muscle cells in bioreactors, producing cultured meat products such as beef, poultry, pork and seafood in the form of burgers, nuggets and fish fillets that closely mimic conventional flesh in taste, texture and nutritional value.20,21 These products have been produced at pilot and pre-commercial scales and are valued for their potential to reduce environmental impact and animal suffering.1,3
Other food-based alternative proteins
Through precision fermentation, microorganisms are engineered to produce animal proteins like casein (milk protein), ovalbumin (egg white protein) and whey protein, which are then purified and used in foods such as animal-free dairy products and egg substitutes. These proteins are already incorporated into commercially available products and offer vegan, allergen-reduced and sustainable alternatives.1,12
Vegan leather
Cellular agriculture enables the production of animal-free leather by cultivating structural proteins like collagen or using biological materials such as fungal mycelium and bacterial cellulose. These materials are processed into leather-like sheets used in shoes, bags and textiles, offering a sustainable alternative to traditional leather. Fungal bioleather, especially from Ganoderma lucidum, stands out for its biodegradability and low-cost production using agricultural waste. The production of lab-grown collagen-based leather and bacterial cellulose leather are still being studied but show promise.22,23
Artificial silk
Cellular agriculture enables the production of artificial silk by engineering microorganisms and transgenic organisms to produce spider silk proteins, offering a cruelty-free and biodegradable alternative to traditional silk. While natural silk, especially from spiders, is difficult to harvest at scale, recombinant production using hosts like bacteria, yeasts, mammalian cells and genetically modified silkworms allows silk fibers to be spun that replicate the strength and flexibility of natural spider silk. These fibers are already being used in textiles and high-performance materials across fashion, medical and industrial applications.23,24
Other applications
Plant cell cultures are being developed to produce wood-like materials by cultivating cambial stem cells from trees in a gel medium that encourages them to grow into rigid structures with similar properties to natural wood.25 Similarly, cultured coffee26 is produced through microbial fermentation, where specific plant cells or microbes are programed to biosynthesize coffee compounds like caffeine and aromatic molecules.
In conclusion, cellular agriculture represents a transformative shift in how we produce food, materials and other biological products. By leveraging cell culture, tissue engineering and precision fermentation, it enables the creation of sustainable, ethical and innovative alternatives to traditional animal and plant agriculture. From cultured meat and dairy proteins to bioleather and functional ingredients made by microalgae, fungi and yeast, the field is already demonstrating its potential to reduce environmental impact, improve food security and meet the growing global demand for ethical and efficient food systems.
However, despite its promise, cellular agriculture still faces technical, economic and regulatory challenges. Scaling up production, reducing costs and gaining consumer acceptance will be critical for its mainstream success. Continued investment in research, transparent communication and policy development will be essential to realize the full benefits of this revolutionary field.
1. Eibl R, Senn Y, Gubser G et al. Cellular agriculture : Opportunities and challenges. Annu Rev Food Sci Technol. 2021;12:51-73. doi:10.1146/annurev-food-063020-123940
2. Wali M El, Golroudbary SR, Kraslawski A, Tuomisto HL. Transition to cellular agriculture reduces agriculture land use and greenhouse gas emissions but increases demand for critical materials. Commun Earth Environ. 2024;5(61):1-17. doi:10.1038/s43247-024-01227-8
3. Rischer H, Szilvay R, Oksman-Caldentey K-M. Cellular agriculture – Industrial biotechnology for food and materials. Curr Opin Biotechnol. 2020;61:128-134. doi:10.1016/j.copbio.2019.12.003
4. Soice E, Johnston J. How cellular agriculture systems can promote food security defining requirements of food security. Front Sustain Food Syst. 2021;5:1-13. doi:10.3389/fsufs.2021.753996
5. Post MJ. Cultured beef : Medical technology to produce food. Soc Chem Ind. 2014;94:1039-1041. doi:10.1002/jsfa.6474
6. Nyika J, Mackolil J, Workie E, Adhav C, Ramadas S. Cellular agriculture research progress and prospects : Insights from bibliometric analysis. Curr Res Biotechnol. 2021;3:215-224. doi:10.1016/j.crbiot.2021.07.001
7. Helliwell R, Burton RJF. The promised land ? Exploring the future visions and narrative silences of cellular agriculture in news and industry media. J Rural Stud. 2021;84:180-191. doi:10.1016/j.jrurstud.2021.04.002
8. Bharatgiri A, Rybchyn MS, Walsh WR, Coutre J le. Obtaining source material for cellular agriculture. Heliyon. 2024;10(18):e38006. doi:10.1016/j.heliyon.2024.e38006
9. Nyysso A, Suhonen A, Ritala A, Oksman-Caldentey K-M. The role of single cell protein in cellular agriculture. Curr Opin Biotechnol. 2022;75:1-7. doi:10.1016/j.copbio.2022.102686
10. Silva M, Geada P, Pereira RN, Teixeira JA. Microalgae biomass – A source of sustainable dietary bioactive compounds towards improved health and well-being. Food Chem Adv. 2025;6:100926. doi:10.1016/j.focha.2025.100926
11. Hilgendorf K, Wang Y, Miller MJ, Jin Y. Precision fermentation for improving the quality , flavor , safety , and sustainability of foods. Curr Opin Biotechnol. 2024;86:103084. doi:10.1016/j.copbio.2024.103084
12. Dupuis JH, Cheung L, Newman L, Dee DR, Yada R. Precision cellular agriculture : The future role of recombinantly expressed protein as food. Compr Rev Food Sci Food Saf. 2023;(22):882-912. doi:10.1111/1541-4337.13094
13. Subbiahanadar K, Durairaj J, Christyraj S, et al. Alternative to FBS in animal cell culture – An overview and future perspective. Heliyon. 2021;7(8):e07686. doi:10.1016/j.heliyon.2021.e07686
14. Charlesworth JC, Jenner A, Coutre J le. Plant-based hydrolysates as building blocks for cellular agriculture. Food Chem. 2024;460:140621. doi:10.1016/j.foodchem.2024.140621
15. Chen L, Guttieres D, Koenigsberg A, Barone PW, Sinskey AJ, Springs SL. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials. 2022;280:121274. doi:10.1016/j.biomaterials.2021.121274
16. Verbruggen S, Luining D, van Essen A, Post MJ. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology. 2018;70(2):503-512. doi:10.1007/s10616-017-0101-8
17. Schüler K, Marques DMC, Gusmão A, et al. 3D printing of plant-based fat inks towards manufacturing complex cellular agriculture products with fatty structures. Food Hydrocoll. 2024;157. doi:10.1016/j.foodhyd.2024.110369
18. Lee SH, Choi J. Three-dimensional scaffolds, materials, and fabrication for cultured meat applications: A scoping review and future direction. Food Hydrocoll. 2024;152:109881. doi:10.1016/j.foodhyd.2024.109881
19. Zhu W, Iskandar MM, Baeghbali V, Kubow S. Three-dimensional printing of foods: A critical review of the present state in healthcare applications, and potential risks and benefits. Foods. 2023;12(17). doi:10.3390/foods12173287
20. Tibrewal K, Dandekar P, Jain R. Extrusion-based sustainable 3D bioprinting of meat & its analogues: A review. Bioprinting. 2023;29:e00256. doi:10.1016/j.bprint.2022.e00256
21. Rubio N, Datar I, Stachura D, Kaplan D, Krueger K. Cell-based fish: A novel approach to seafood production and an opportunity for cellular agriculture. Front Sustain Food Syst. 2019;3:1-13. doi:10.3389/fsufs.2019.00043
22. García C, Prieto MA. Bacterial cellulose as a potential bioleather substitute for the footwear industry. Microb Biotechnol. 2019;918373112(4):582-585. doi:10.1111/1751-7915.13306
23. Kamble P. Cellular agriculture : The way ahead for food , textile , and cosmetic industries. Int J Adv Res Ideas Innov Technol. 2021;7(4):1539-1546.
24. Burton RJF, Campbell H. The impact of “artificial wool” on the New Zealand wool industry: Lessons for future substitution transitions in the agricultural sector. J Rural Stud. 2025;114:103578. doi:10.1016/j.jrurstud.2025.103578
25. Dinneen J. Sliver of wood grown from stem cells. New Sci. 2024;264(3519):10. doi:10.1016/S0262-4079(24)02091-8
26. Aisala H, Kärkkäinen E, Jokinen I, Seppänen-Laakso T, Rischer H. Proof of concept for cell culture-based coffee. J Agric Food Chem. 2023;71(47):18478-18488. doi:10.1021/acs.jafc.3c04503