Boost FDA Modernization Act 2.0 Readiness With an Advanced LIMS
The FDA Modernization Act 2.0 helps labs move from animal testing to human-relevant models.

April was a remarkable month for both the scientific and regulatory communities in clinical research. The FDA finally set concrete plans for implementing the FDA Modernization Act 2.0, bringing us steps closer to the end of mandatory animal testing in preclinical studies. In the same month, the NIH launched an initiative to prioritize human-based research technologies over animal-based research technologies. The NIH noted that human-based research, either in isolation or in combination with animal research, will help to provide more clinically relevant results. These changes reflect growing concerns about the limitations of animal models and the need for more human-relevant testing methods.
For example, a 2023 review highlighted how more than 90% of drugs that pass animal studies ultimately fail in human trials due to unexpected toxicity or lack of efficacy. Such failures are often linked to the fundamental biological differences between animals and humans, among other reasons.
Let’s discuss what the FDA Modernization Act means for clinical research labs, the rise of alternative test methods and the role that a Laboratory Information Management System (LIMS) plays in ensuring compliance and seamless workflows in clinical research.
The FDA Modernization Act 2.0 is a revision of the 21st century FDA Modernization Act (FDAMA), which was passed in 1997. This is also an improvement of the original Federal Food, Drug and Cosmetic Act that was passed in 1938. The fundamental role of this act is to ensure the safety of food, drugs, medical devices and biological products by mandating a pre-market review and approval process.
For decades, this act required that new drugs undergo compulsory animal testing before advancing to human clinical trials. This was to ensure that human subjects were only exposed to new drugs and devices when some level of safety and efficacy had already been established. Fair enough. However, several issues have arisen over time, including:
- Poor translatability of results from animals to humans
- Ethical concerns over the use “or abuse” of animals for research that only benefits humans
- Difficulty in modeling new drug modalities like monoclonal antibodies in animals
- Inefficiency in terms of time and cost
With these concerns in mind, the FDA was prompted to make some changes – hence the revision of FDAMA which later led to the Modernization Act 2.0. The new act, passed towards the end of 2022, offers clinical researchers “non-animal” alternatives that include the following:
- In vitro systems, such as microphysiological systems (MPS) to simulate organ-level function using human cells
- Organoids and tissue chips, which are miniature three-dimensional models that replicate specific tissue structures in controlled lab settings
- In silico alternatives that include AI-based simulations and predictive modeling, such as Physiologically-Based Pharmacokinetic (PBPK) modeling, Quantitative Systems Pharmacology (QSP) and modeling of biological pathways
- Cell-based assays that use human cells in lab settings to allow for biologically more accurate testing
- Computational tools and advanced analytics to interpret complex safety and efficacy data
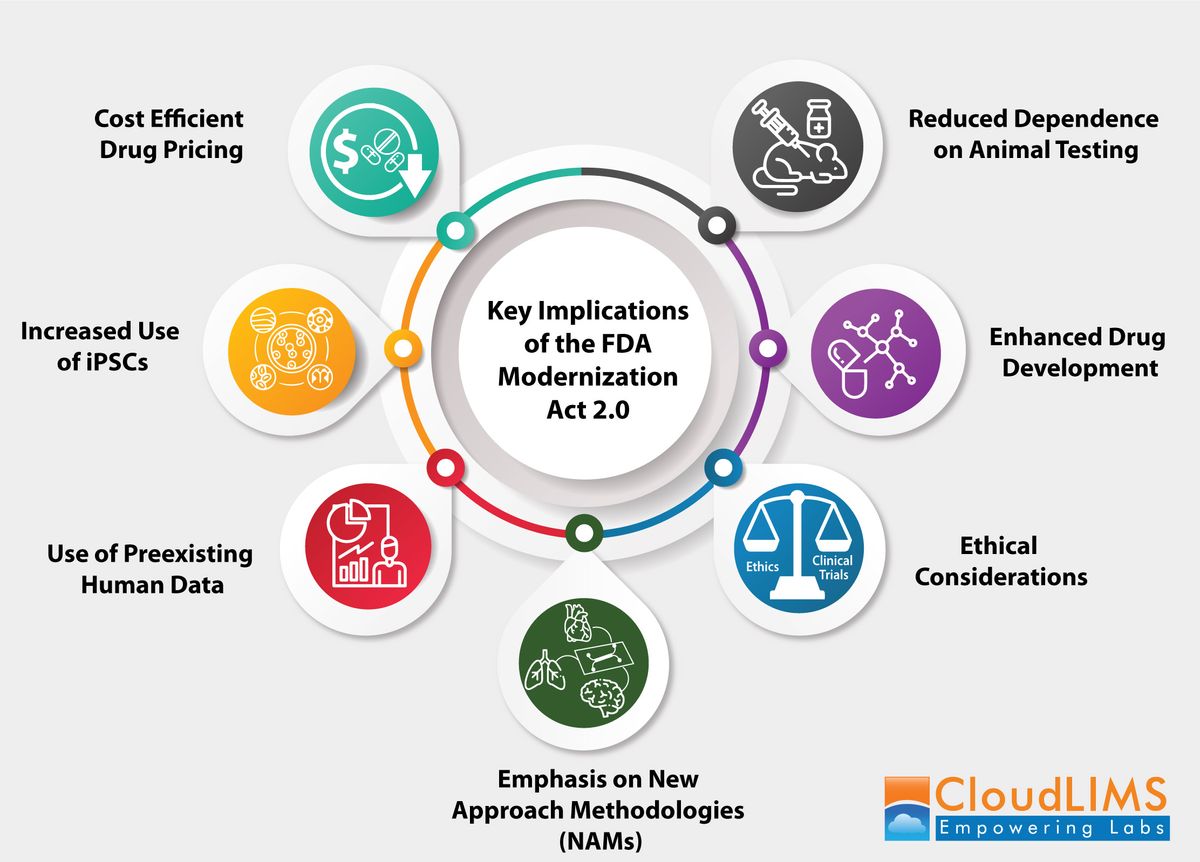
Schematic representation of the key impacts of the FDA Modernization Act 2.0 on clinical research laboratories. Credit: CloudLIMS.
Key implications of the FDA Modernization Act 2.0 for clinical research laboratories
Reduced dependence on animal testing
Animal testing has been the mainstay of preclinical research, but this has not been without setbacks. A major issue is the poor translatability of results, leading to high attrition rates in drug development. The FDA Modernization Act 2.0 provides suitable alternatives that use human biology from the outset. This approach is not only more ethical but also more realistic in terms of clinical translatability.
Emphasis on new approach methodologies
This new act prioritizes the use of new approach methodologies (NAMs) including MPS (such as organ-on-a-chip), computational models and cell-based assays for preclinical research. The aim is to accelerate the research process as well as to improve the reproducibility of results in subsequent studies.
Enhanced drug development
Animal-based research, while helpful, often delays the research process, especially when results fail to translate in human trials. The newer methods use human-relevant models which are likely to solve this problem and hence improve the success rate of clinical trials.
Animal rights advocates have long opposed the use of animals in research designed primarily to benefit humans. This new act provides viable alternatives to reduce, refine or replace animal use in research.
Use of preexisting human data
For many years, the FDA has mandated drug manufacturers to conduct animal testing for drugs that already have broad human use safety and efficacy data internationally. This is no longer the case as data (from human studies) from countries with comparable regulatory standards can now be used.
Increased use of induced pluripotent stem cells
The new act encourages the adoption of induced pluripotent stem cells (iPSCs), an alternative that offers physiologically relevant models with greater potential for clinical translatability.
Cost-efficient drug pricing
Animal-based research is often expensive both in terms of time and cost due to high failure rate. Adoption of NAMS is likely to translate to a significant reduction in R&D costs, ultimately leading to more affordable drug pricing.
Implementation of the FDA Modernization Act 2.0 is an ongoing process. Over time, and as regulators become more confident in the reliability of NAMs, the product approval journey will become more efficient. This will ultimately benefit not only researchers and sponsors but most importantly patients in dire need of these treatments.
In April 2025, the FDA released a phased approach to the adoption of scientifically validated NAMS as a foundational part of clinical research. It also stipulates collaboration methods between different stakeholders in making this transition a success. The roadmap is designed to commence with monoclonal antibodies (mAbs), identified as a promising avenue for reducing animal use in preclinical safety testing. Subsequently, its scope will broaden to encompass additional biological molecules, followed by new chemical entities and medical countermeasures.
For laboratories that support clinical research, this has a number of implications. It means handling an influx of diverse data types from different instruments and software as well as keeping up with a host of regulations.
How can a modern lab keep up with the demands of the FDA Modernization Act 2.0?
Leverage an advanced LIMS for best outcomes
The FDA Modernization Act introduces significant changes in laboratory processes, especially when labs need to adapt to advanced models such as MPS. At the same time, they must also keep up with new regulatory requirements for these new testing models.
How can a LIMS help?
The first role that a clinical research LIMS plays is to automate lab workflows; this enables the lab to handle more sample volumes efficiently. A configurable LIMS makes it easy for labs to adapt their workflows to new testing methodologies without any operational disruptions. By integrating seamlessly with lab instruments and software systems, a LIMS eliminates the need for manual data transfers, hence reducing the risk of errors and data silos.
An advanced, cloud-based LIMS adds further value by providing centralized access to trial data and sample information. This makes it easier for remote teams to collaborate. It also simplifies the tracking of protocol deviations, and maintains a secure audit trail to make regulatory compliance easier. For laboratories adapting to the FDA Modernization Act 2.0, a LIMS provides the structure, flexibility and control needed to keep up with the emerging demands.
With the rollout of the FDA Modernization Act 2.0, and the phasing out of compulsory animal studies, clinical research is bound to be more ethical, efficient and successful in translating preclinical findings into human trials. This shift can only be successful if clinical research laboratories are on board. With an advanced, configurable and cloud-based LIMS, these labs can easily keep up with new workflows, while confidently supporting the evolving demands of the FDA Modernization Act 2.0.